
| Friends of Five Creeks |
Water Quality Monitoring: Nitrates
Nitrates
- Nitrogen occurs in natural waters as nitrate (NO3), nitrite (NO2), ammonia (NH3), and organically bound nitrogen.
- As aquatic plants and animals die, bacteria break down large protein molecules containing nitrogen into ammonia. Ammonia is then oxidized by specialized bacteria to form nitrites and nitrates.
- Sewage is the main source of nitrates added by humans to rivers. Another important source is fertilizers, which can be carried into creeks by stormwater runoff.
- Excessive nitrates stimulate growth of algae and other plants, which later decay and increase biochemical oxygen demand as they decompose.
Test Equipment
- Hach Low Range Nitrate Test Kit (Cat. No. 14160-00)
- Wash bottle containing demineralized water
- Cadmium waste jar
- Gloves and goggles
- Waste container
Procedure
The color comparison performed at the end of the procedure can be difficult if the sample water is colored or if the reagents produce a hue that does not closely match the color wheel. Cerrito Creek is often slightly yellow, for example. The treated sample can be compared to one or more of several reference points including distilled water, untreated sample, and reagent blank (i.e., treated distilled water). Please note on the field data sheet which reference point is used and any difficulties in comparison. If comparing to a reagent blank, please also compare the reagent blank to distilled water and record the offset.Nitrates
- Put on gloves and goggles. Fill one of the color viewing tubes part way (half, for example) with sample water. Stopper the tube and shake vigorously. Empty the tube and repeat this procedure once.
- Fill the color viewing tube to the 5 ml mark with the sample. The mark is located at the bottom of the frosted marking area, approximately 2.5 cm from the bottom of the viewing tube.
- Open one NitraVer 6 nitrate reagent powder pillow. Add the contents of the pillow to the sample to be tested. Stopper the tube and shake for three minutes. Allow the sample to stand undisturbed for an additional 30 seconds. Unoxidized particles of cadmium metal will remain in the sample and settle to the bottom of the viewing tube.
- Pour the prepared sample into a second color viewing tube carefully so that the cadmium particles remain in the first tube.
- Open one NitriVer 3 nitrite reagent powder pillow. Add the contents of the pillow to the sample. Stopper the tube and shake for 30 seconds. A red color will develop if nitrate is present. Allow at least 10 minutes, but not more than 20 minutes, before completing steps 6 through 8.
- Insert the tube of prepared sample into the inside top opening of the color comparator.
- Rinse the unoxidized cadmium metal from the color viewing tube used in step 2 with the wash bottle and pour it into the cadmium waste jar. Fill the color viewing tube to the mark with an untreated water sample (or distilled water or reagent blank) and place in the outside top opening of the comparator. The color of this sample will serve as a reference point.
- Hold the comparator up to a light source such as the sky, a window or lamp and view through the openings in front. Rotate the disc to obtain a color match. Read the ppm nitrate nitrogen through the scale window. Make a note in the comments section of the field data sheet explaining which reference point was used. To obtain the results as ppm nitrate, multiply the reading on the scale by 4.4.
Clean-up
- Pour prepared and untreated water samples into the waste container.
- Return all glassware and chemicals to their boxes.
- When safe to do so, remove gloves and goggles.
Water Quality Index
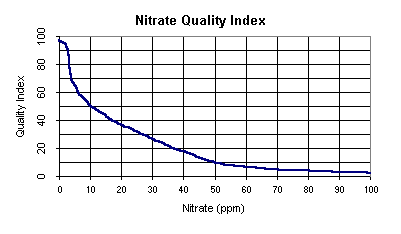
Note: If nitrate nitrogen is greater than 100 ppm,
the quality index equals 1.
Calculations
- Convert nitrates (ppm) to water quality index.